Objective
- To study reaction of base with metal
Materials required:
- Sodium hydroxide solution
- Some pieces of aluminium metal
- red and blue litmus papers
- three test tubes
- candle
Procedure:
- Take a small piece of aluminium metal and place it in a clean and dry test tube
- Add about 5 mL sodium hydroxide solution in it
- Observe the changes in the test tube

Findings:
|
Test |
Activity |
Observation |
|
Color |
Look at the colour of the gas liberated |
Colorless |
|
Smell |
Fan the gas gently towards your nose |
Odorless |
|
Litmus test |
Bring moist blue and red litmus papers near to the mouth of the test tube |
No change |
|
Combustion test |
Bring a lighted candle near to the mouth of the test tube |
Burn with pop sound |
Conclusions:
- The reaction between sodium hydroxide solution and hydrochloride acid is exothermic
- Reaction between barium hydroxide solution and ammonium chloride is endothermic.
Theory:
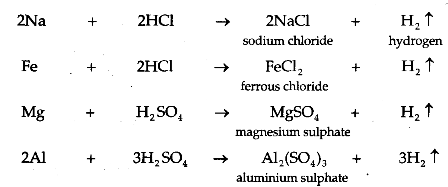
- When alkali (base) reacts with metal, it produces salt and hydrogen gas
- Example: Sodium hydroxide gives hydrogen gas and sodium zincate when reacts with zinc metal. Sodium aluminate and hydrogen gas are formed when sodium hydroxide reacts with aluminium metal.