Turn a Potato Into a Battery
Abstract
Imagine telling your friends about your latest science project: using a battery to make a light turn on. You might get some blank stares...sounds a little boring and basic, right? Now tell them you will do it with a potato! Yes, you can actually use fruits and vegetables as part of an electric power source! Batteries power many things around you, including cell phones, wireless video game controllers, and smoke detectors. In this science project, you will learn about the basics of battery science and use potatoes to make a simple battery to power a small light and a buzzer.
Summary
AREAS OF SCIENCE
Recommended Project Supplies

Objective
Measure how the voltage and current of potato batteries change when you combine them in series or parallel..
Introduction
Batteries are containers that store energy, which can be used to make electricity. This method of storing energy allows us to make portable electronic devices (imagine what a pain it would be if everything had to be plugged into a wall outlet to work!). There are many different types of batteries, but they all depend on some sort of chemical reaction to generate electricity. The chemical reaction typically occurs between two pieces of metal, called electrodes, and a liquid or paste, called an electrolyte. It turns out that the moisture inside a potato works pretty well as an electrolyte, so you just need to add some metal electrodes to a potato, and you have a battery! While you do not need to understand the details of the chemical reaction to do this project, it is explained in technical note at the end of this introduction.
Next, you need to understand some basic concepts about electricity. The flow of electricity is called an electrical current, which is measured in a unit called amperes (A) (also called amps for short). Voltage, measured in volts (V) is what pushes electrical current through wires. Finally, electrical resistance, measured in ohms (Ω) (the capital Greek letter Omega) is a measure of how difficult it is for electricity to flow through a certain material. A common analogy for electricity is to imagine water flowing through a pipe. The amount of water flowing is like the current. The pressure pushing the water is like the voltage. The resistance is like the size of the pipe—it is harder to squeeze a lot of water quickly through a very tiny pipe than through a big pipe. Does that seem like a lot to remember? Table 1 summarizes voltage, current, and resistance.
| Quantity | Unit | Description |
|---|---|---|
| Current | Ampere (A) | The "flow" of electricity |
| Voltage | Volt (V) | The "pressure" that makes electricity flow |
| Resistance | Ohm (Ω) | How hard it is for electricity to flow through something |
An electrical circuit is a path through which the electricity can flow. Circuits can be very complex, with millions and millions of components (like the ones inside your computer), or very simple, with just two components, like a battery and a lightbulb. This project will focus on simple battery-powered circuits. In general, a battery supplies a certain voltage to a circuit. How much current is drawn out of the battery depends on the load, or what the battery is connected to.
Batteries have positive and negative terminals. In order for electricity to flow in a battery-powered circuit, there must be a complete path from the positive terminal, through the load, and back to the negative terminal. This is called a closed circuit. If the path is broken (for example, if one wire is disconnected), electricity cannot flow. This is called an open circuit. Finally, if there is a direct path from the positive to the negative terminal, this is called a short circuit. Short circuits are bad because they can cause batteries to drain very quickly and overheat (luckily, potato batteries can only supply a small amount of current, so short circuits in this experiment are not dangerous). Figure 1 shows diagrams of open, closed, and short circuits.
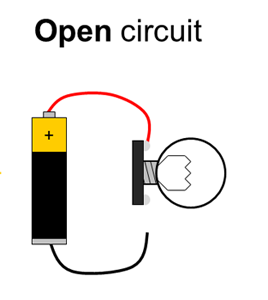
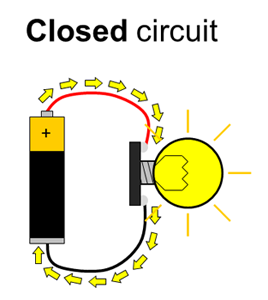
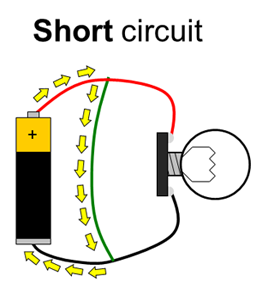
Figure 1. Closed, open, and short circuits. Electrical current is represented by the yellow arrows. In the open circuit, no current flows at all. In the closed circuit, current flows through the lightbulb, causing it to light up. In the short circuit, current flows directly between the battery terminals, bypassing the lightbulb, so it does not light up.
What about circuits that have more than just a single battery? You have probably used many devices that require two or more batteries, like toys or remote controls. Multiple batteries can be connected two different ways: in series or in parallel. When multiple batteries are connected in series, the positive terminal of one battery is connected to the negative terminal of the next battery (and this repeats if there are more than two batteries). When batteries are connected in parallel, all of the positive battery terminals are connected together, and all of the negative battery terminals are connected together. These two configurations are shown in Figure 2.
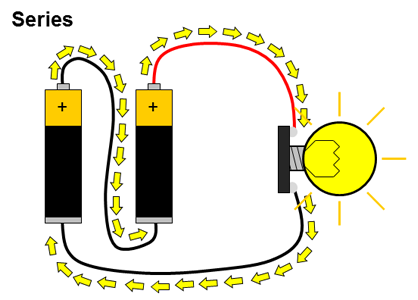
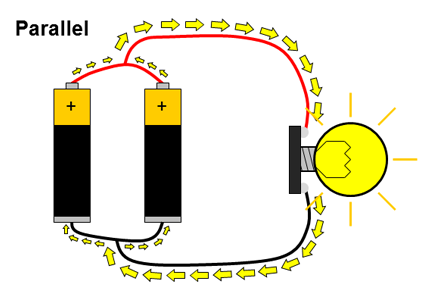
Figure 2. Batteries connected in series and parallel. Electrical current is represented by the yellow arrows.
So why would you choose one method over the other? The amount of voltage and current that can be supplied by multiple batteries changes depending on whether you connect them in series or in parallel, and certain electronic devices might require a certain amount of voltage or current. For example, have you ever noticed how a small device like a TV remote or computer mouse might only require two AAA batteries, but a larger toy or flashlight might require four or more AA batteries? This is because each device has different electrical requirements to operate properly.
You can measure how much voltage or current a certain number of batteries can provide by determining the batteries' open-circuit voltage and short-circuit current. A battery's open-circuit voltage is the voltage across a battery's terminals when it is not attached to anything. This is the highest voltage that a battery can supply (the voltage will drop slightly when the battery is attached to a load). The short-circuit current is the current when the battery's terminals are shorted together. This is the highest current the battery can supply (the current will also drop when the battery is attached to a load). How exactly do the voltage and current change when your batteries (potatoes) are configured in series or in parallel? In this project, you will use a multimeter, a device that can measure electrical circuits, to find out.
In a battery, chemical energy is converted into electrical energy. In general, electrical current consists of the flow of electrons, which are negatively charged particles. In a potato battery, the electrical energy is generated by two chemical reactions that happen at the electrodes (the copper and zinc metal strips). Because copper is more electronegative than zinc, it tends to attract electrons more easily than zinc. Therefore, electrons in a potato battery will flow from the zinc electrode, the anode, to the copper electrode, the cathode. But where do these electrons come from? The electrons are derived from the zinc metal. When zinc is in contact with the electrolyte, the following oxidation reaction takes place which results in the release of electrons:
Equation 1 (zinc electrode):While the the zinc joins the electrolyte as a positive ion (Zn++), the electrons flow through the wire connecting the electrodes (Figure 3 shows an LED connected between the electrodes, but as you will see in the procedure, this could also be a multimeter or a buzzer). Here, positively charged hydrogen atoms, or protons (H+), that originate from acids inside the potato (phosphoric acid and organic acids), accumulate as they are attracted to the negative charge created by the surplus electrons at the copper electrode. These protons take up the electrons from the copper electrode in a reduction reaction to become neutral hydrogen atoms and subsequently form hydrogen gas (H2) which you might see as bubbles evolving around the copper electrode:
Equation 2 (copper electrode):This reaction leaves behind a shortage of electrons at the copper electrode, which is the key reason why electrons keep flowing from the zinc to the copper electrode. The battery keeps running until it either runs out of protons if there is not a lot of acid present, or the zinc electrode is "eaten up" and completely dissolved into ions. The overall net reaction in a potato battery can be summarized as:
Equation 3 (net reaction):Note that the potato itself is not the sole energy source for the battery, but functions as an electrolyte to facilitate the transport of relevant ions such as zinc cations and protons that originate from organic acids and phosphoric acid that are present inside the potato.
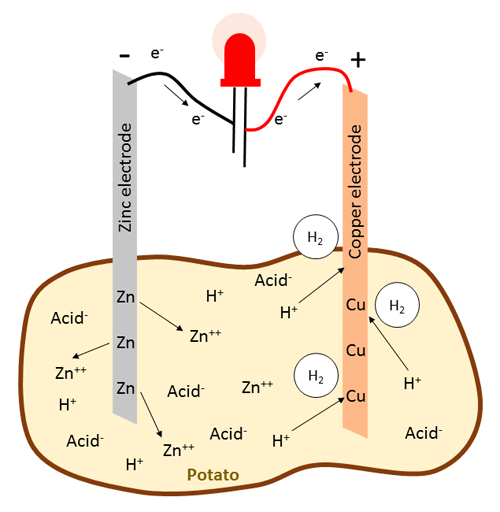
Figure 3. Diagram of the chemical reaction that occurs in a potato battery, with an LED connected between the electrodes. Note that the arrows representing electron movement in this figure point in the opposite direction as those that represent electrical current in Figures 1–2.
Terms and Concepts
- Battery
- Chemical reaction
- Electrode
- Electrolyte
- Current
- Ampere
- Amp
- Voltage
- Volts
- Resistance
- Ohms
- Circuit
- Load
- Terminal
- Closed circuit
- Open circuit
- Series circuit
- Parallel circuit
- Open-circuit voltage
- Short-circuit current
- Multimeter
Advanced terms:
- Electron
- Electronegative
- Anode
- Cathode
- Oxidation
- Reduction
Questions
- What are the basic parts of a battery? How do batteries generate electrical current?
- What is electrical current? What is its unit of measurement?
- What is electrical voltage? What is its unit of measurement?
- What is electrical resistance? What is its unit of measurement?
- What are the differences between open, closed, and short circuits?
- How is the flow of electricity similar to the flow of water?
- If one vegetable or fruit battery is not enough to power a buzzer, what could you do to resolve the problem?
Materials and Equipment
Recommended Project Supplies
- Veggie Power Battery Kit, available from our partner Home Science Tools. Includes:
- Copper electrodes (3)
- Zinc electrodes (3)
- Alligator clip leads (6)
- Digital multimeter with test leads
- Piezoelectric buzzer
- Red light-emitting diode (LED) (3)
- You will also need to gather these items, not included in the kit:
- Potatoes (3), any large type like a russet. Make sure your potatoes are fresh. Old, dried out potatoes will not work well.
- Ruler
- Paper towels for cleanup as you prepare the potatoes
- Lab notebook
