Objective
Identification of the acidic/basic/neutral nature of the salt solutions
Materials required:
- Ferric chloride
- Sodium acetate
- Sodium chloride
- Test tubes
- Dropper
- Litmus papers
Procedure:
- Take about 0.5g of ferric chloride solution (in a test tube and add 10ml of water to it.
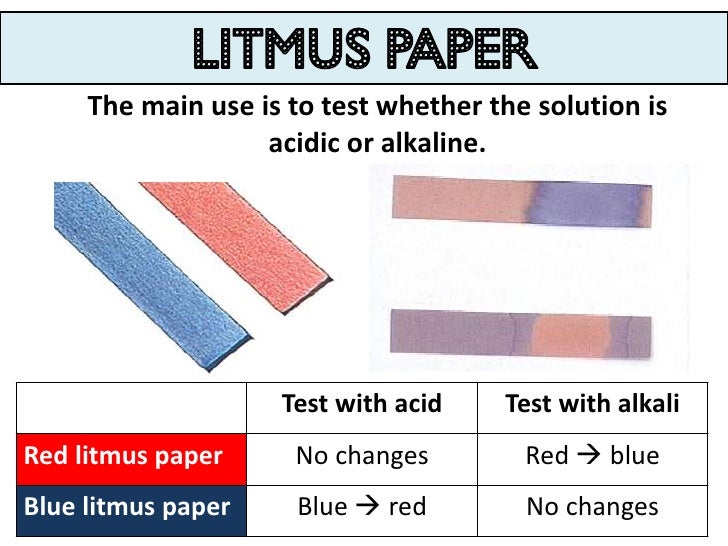
- Take a piece of blue litmus paper and dip it in this solution
- Dip a piece of red litmus paper in the solution and observe the change
- Repeat the above steps with sodium acetate solution and sodium chloride solution, respectively. Note the observations
Findings:
- Ferric chloride solution turns blue litmus paper red, but it does not change the colour of red litmus paper
- Sodium acetate solution turns red litmus paper blue but does not change the colour of blue litmus paper
- Sodium chloride solution does not change the colour of either red or blue litmus paper
Conclusions:
- Ferric chloride solution is acidic
- Sodium acetate solution is basic
- Sodium chloride solution is neutral
Theory:
- Ferric Chloride dissolves in water to make Ferric Hydroxide (a weak base) and Hydrochloric Acid (a strong acid). Resultant solution is acidic in nature.
- Sodium Acetate solution is basic
- Sodium Chloride is a salt of strong acid (HCl) and Strong Base (NaOH), it is neutral

- ACID TURNS BLUE LITMUS RED; BASE TURN RED LITMUS BLUE