Experiment 22: Reactivity of metals
Objective
- Study reactivity of metals
Materials required:
- Pieces of metals such as zinc, copper, iron, and Aluminium or other suitable metals (at least four strips of each metal)
- solutions like zinc sulphate; copper (II) sulphate; iron (II) sulphate; and Aluminium chloride
- distilled water
- Measuring cylinder
- Test tubes and test tube stand
Procedure:
- Take zinc, copper, iron, and aluminium metal pieces and clean their surfaces
- Prepare 20mL solutions of 5% concentration (by volume) of zinc sulphate, copper (II) sulphate, iron (II) sulphate and aluminium chloride in distilled in four different beakers. Label these beakers as W, X, Y, and Z. Note that these are the salt solutions of the four metals taken for studying the interaction
- Take 10 mL of each solution in four different test tubes and label them as tubes A, B, C, and D
- Put zinc metal strip in all the four test tubes, that is in tubes A, B, C, and D and observe the change that follows
- Repeat the above experiment with other metal strips by dipping them in fresh salt solutions of metals and observe for displacement reactions

Findings:
|
Metal |
Zinc Sulphate (A) |
Copper Sulphate (B) |
Ferric chloride (C) |
Al Chloride (D) |
|
Zinc |
||||
|
Copper |
||||
|
Iron |
||||
|
Aluminium |
Conclusions:
- Arrange the metals in the order of their decreasing reactivity
Theory:
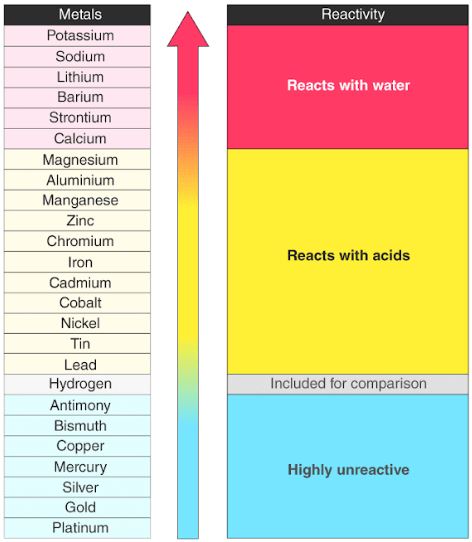
- Metals tend to readily lose electrons and form cations. Most of them react with atmospheric oxygen to form metal oxides. However, different metals have different reactivities towards oxygen (unreactive metals such as gold and platinum do not readily form oxides when exposed to air).
- The metals at the top of the reactivity series are powerful reducing agents since they are easily oxidized. These metals tarnish/corrode very easily.
- The reducing ability of the metals grows weaker while traversing down the series.
- The electro positivity of the elements also reduces while moving down the reactivity series of metals.
- All metals that are found above hydrogen in the activity series liberate H2 gas upon reacting with dilute HCl or dilute H2SO4.
- Metals that are placed higher on the reactivity series have the ability to displace metals that are placed lower from their salt solutions.
- Higher ranking metals require greater amounts of energy for their isolation from ores and other compounds.