Objective
Test the presence of proteins in common food items (Biuret test)
Materials required:
- Food items: Soaked chickpea seeds, Banana
- Dropper
- Test tubes
- Copper Sulphate solution
- Caustic soda (Sodium Hydroxide solution)
Procedure:
- Grind 10-15 seeds of gram or pea into powder form; and mash a piece of banana separately to form a paste
- Take a small quantity of these food items in the separate test tubes and label them ‘A’ and ‘B’
- Add 10-15 drops of water to each test tube
- With the help of droppers, add 2-3 drops of copper sulphate solution and 10 drops of caustic soda to each test tube
- Shake well and keep the test tubes aside for a few minutes.
- Note the change in colour and record your observations
Findings:
- Contents of test tube ‘A’ containing powdered seeds of gram or pea turn violet in colour whereas test tube ’B’ containing mashed banana does not show colour change.

Conclusions:
- Appearance of violet colour in test tube ‘A’ confirms that gram or pea seeds contain proteins. As banana does not contain proteins, the test tube ‘B’ does not show violet colour. Proteins are body-building components of the food.
Theory:
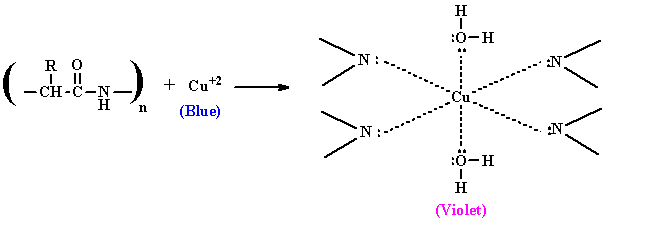
- Biuret test is a general test for compounds having a peptide bond. Biuret is a compound formed by heating urea to 180° C. When biuret is treated with dilute copper sulfate in alkaline condition, a purple colored compound is formed. This is the basis of biuret test widely used for identification of proteins and amino acids.
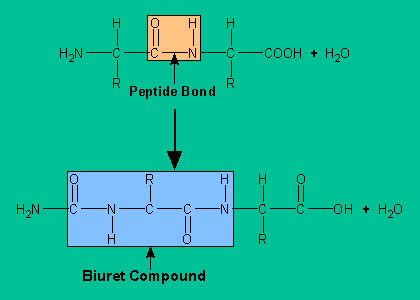
- This test is given by compounds containing two or more peptide bond (CO-NH group). Since all proteins and peptides possessing at least two peptide linkage ie. tripeptide gives positive biuret test.
- The principle of biuret test is conveniently used to detect the presence of proteins in biological fluids
- Alkaline CuSO4 reacts with compounds containing two or more peptide bonds to give a violet colored product which is due to formation of co-ordination complex of cupric ions with un-shared electron pairs of peptide nitrogen and O2 of water.